
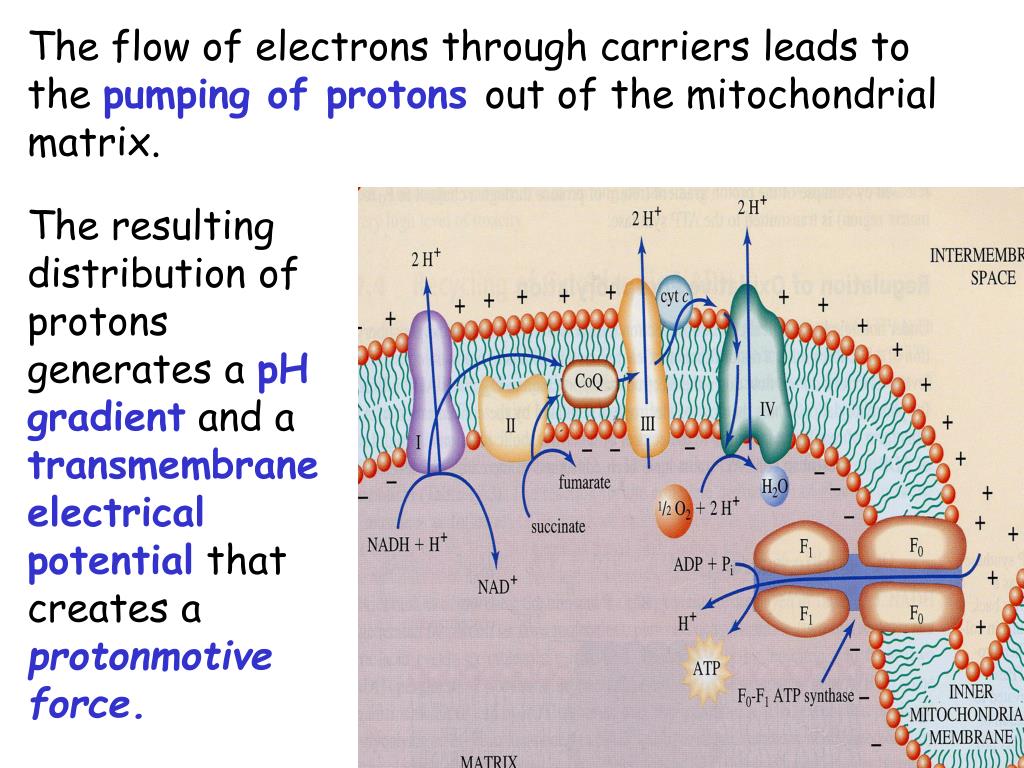
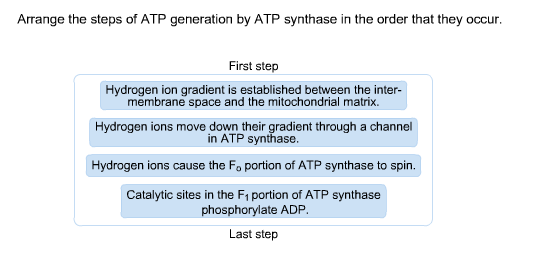
In summary, FOF1ATPase (or synthase) is a rotary enzyme that ultimately couples collapse of a proton gradient (a chemical potential gradient which contributes to the transmembrane electrical potential) to a chemical (phosphorylation) step. M olecular Movies (scroll down to ATP synthase).
This leads to changes in ATP affinity through cycling each through the L, O, and T conformations.įigure: Coupling Proton Flow in F0 to Conformation Change Since the c12 oligomer contacts the γ subunit connecting the Fo stalk and F1 ATPase units, the γ subunit rotates, leading to sequential conformational changes in each of the 3 contacted (αβ)2 dimers of the F1 enzyme. This leads to changes in c subunit interactions which seems to ratchet the c12 core. When a proton is passed to the unprotonated Asp 61, a conformational change in the protonated c subunit occurs. A set of polar residues entirely within subunit a, including Gln 252, Asn 214, Asn 148, Asp 119, His 245, Glu 219, Ser 144 and Asn 238 provide the path as illustrated below. Protons from the inner membrane space or in the periplasmic space (in the above figures) then flow from the periplasm by forming a "handshaking" proton transfer relay which delivers another proton to the deprotonated Arg 210 allowing the circular ratching of the c subunits in the membrane to continue.
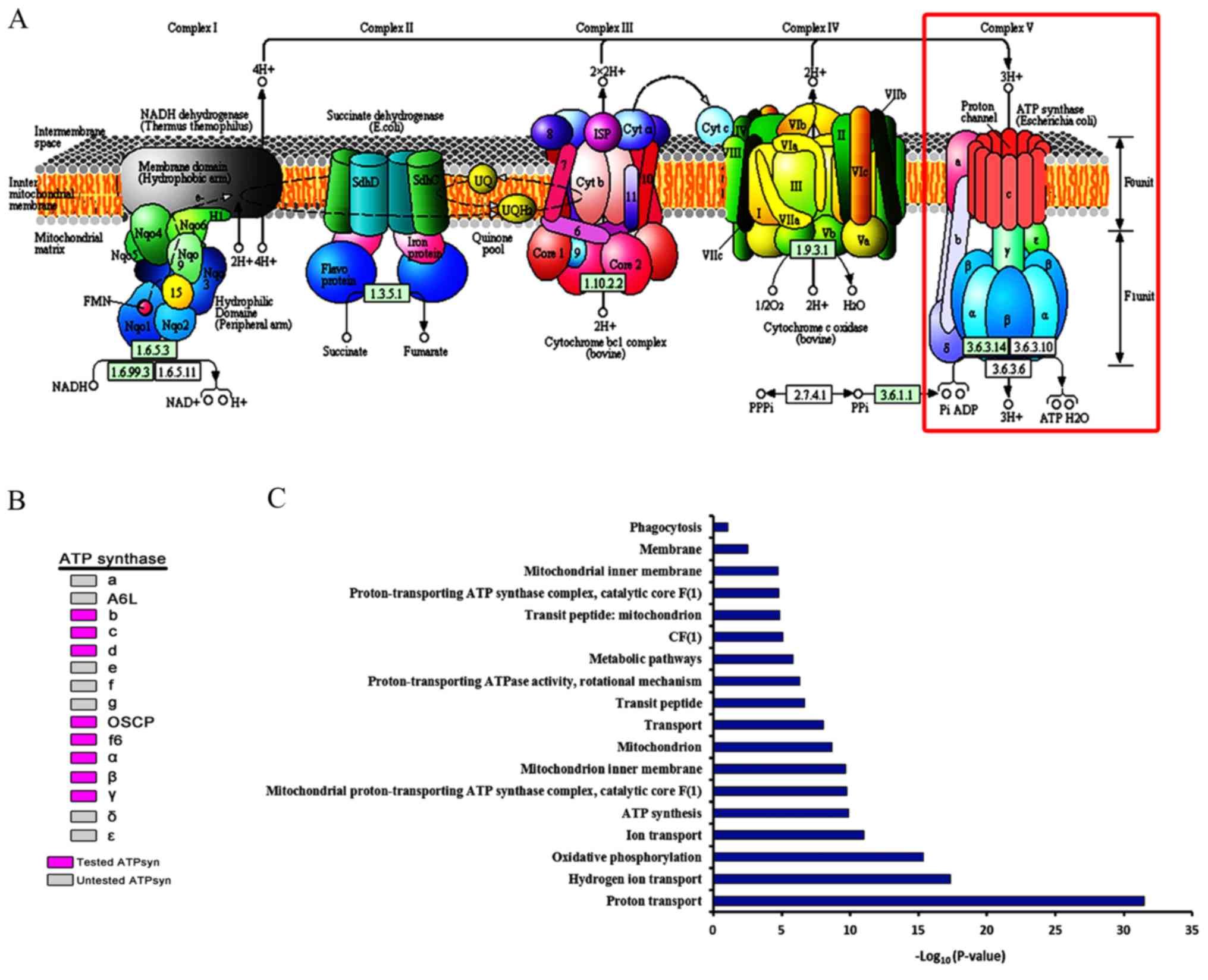
One of the Asp 61 is protonated allowing it to alter conformation and essentially rachet in the membrane domain in a motional faciliated by the development of a neutral protonated Asp. Protons flow to the a chain Arg 210 which is between two Asp 61 on adjacent c chains. The figures below shown the structure of the ac complex from E. The protonated carboxyl group donates a proton to a N atom in DCCD, which then reacts with the deprotonated Asp to form an O-acyl isourea derivative.įigure: Structure of Oligomycin A and DCCD - Inhibitors of proton transport by Fo The modification of one As 61 in only one c subunit is necessary to stop Fo activity. This might occur if the Asp is a very hydrophobic environment. It does so even at pH 8.0 which indicates that the pKa of the Asp 61 is much higher than usual. Another inhibitor, dicyclohexylcarbodiimide reacts with a protonated Asp 61 in c subunits of F0. Oligomycin A sensitivity requires, paradoxically, OSCP (Oligomycin-Sensitivity Conferring Protein which is analogous to the bacterial delta subunit), a stalk protein subunit distal to Fo which couples Fo and F1. One, oligomycin A, binds between the a and c subunits and blocks proton transport activity of the Fo subunit. Two classic inhibitors (structures shown below) of ATP synthase interact with the Fo subunit. The multiple c subunits consist of two very hydrophobic helices connected by a loop in a helix-loop-helix motif. These subunits reside in the inner membrane of the mitochondria (or cell membrane of bacteria) and are involved in proton transport from matrix (or cytoplasm of a bacteria) to the inner membrane space (or periplasmic space of bacteria). A more detailed image of the whole ATO synthase complex is shown below.įigure: Detailed View of F0F1 ATP synthase structureĬloser views of the c subunits and a yellow rectangle representing the a subunit (missing in the combined crystal structure) comprise the Fo part of the complex are shown below. The mechanism by which the proton gradient drives ATP synthesis involves a complex coupling of the F0 and F1 subunits.


 0 kommentar(er)
0 kommentar(er)
